Question: Fuel cell automotive systems are being considered that will require hydrogen of 95% purity. A refinery stream of 800,000 scfm (at 32 F, 1
Fuel cell automotive systems are being considered that will require hydrogen of 95% purity. A refinery stream of 800,000 scfm (at 32οF, 1 atm), containing 72.5% H2, 25% CH4, and 2.5% C2H6, is available. To convert this gas to the required purity, oil absorption, activated charcoal adsorption, and membrane separation are being considered. For oil absorption, an available n-octane stream can be used as absorbent. Because the 95% H2 must be delivered at not less than 375 psia, it is proposed to operate the absorber at 400 psia and 100οF. If at least 80% of the hydrogen fed to the absorber is to leave in the exit gas, determine the:
(a) Minimum absorbent rate in gpm;
(b) Absorbent rate if 1.5 times the minimum amount is used;
(c) Number of theoretical stages;
(d) Stage efficiency for each of the three species in the feed gas, using the O’Connell correlation;
(e) Number of trays actually required;
(f) Exit gas composition, accounting for octane stripping; and
(g) If the lost octane in part
(f) Is not recovered, estimate its value if the process operates 7,900 h/year and the octane is valued at $1.00/gal. Would the octane preclude use of this hydrogen in fuel cells?
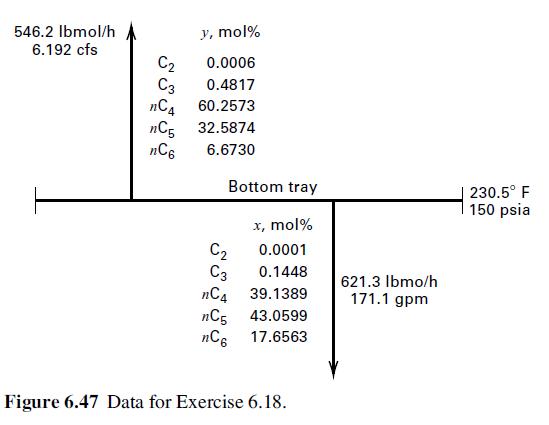
546.2 lbmol/h 6.192 cfs y, mol% 0.0006 C3 0.4817 nC4 60.2573 C2 nC5 32.5874 nC6 6.6730 Bottom tray C C3 nC4 nC5 nC6 x, mol% 0.0001 0.1448 39.1389 43.0599 17.6563 Figure 6.47 Data for Exercise 6.18. 621.3 lbmo/h 171.1 gpm 230.5 F 150 psia
Step by Step Solution
3.44 Rating (170 Votes )
There are 3 Steps involved in it
a Minimum absorbent rate in gpm The minimum absorbent rate can be calculated using the formula Q L x D x S x K where Q is the minimum absorbent rate in gpm L is the molar flow rate of the feed gas in ... View full answer

Get step-by-step solutions from verified subject matter experts


