Question: In Example 15.6, pure-component, liquid-phase adsorption data are used with the extended-Langmuir isotherm to predict a binarysolute data point. Use the mixture data below to
In Example 15.6, pure-component, liquid-phase adsorption data are used with the extended-Langmuir isotherm to predict a binarysolute data point. Use the mixture data below to obtain the best fit to an extended-Langmuir–Freundlich isotherm of the form
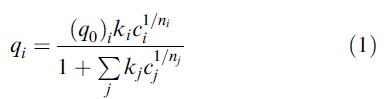
Data for binary-mixture adsorption on activated carbon (1,000 m2/g) at 25°C for acetone
(1) And propionitrile
(2) Are:
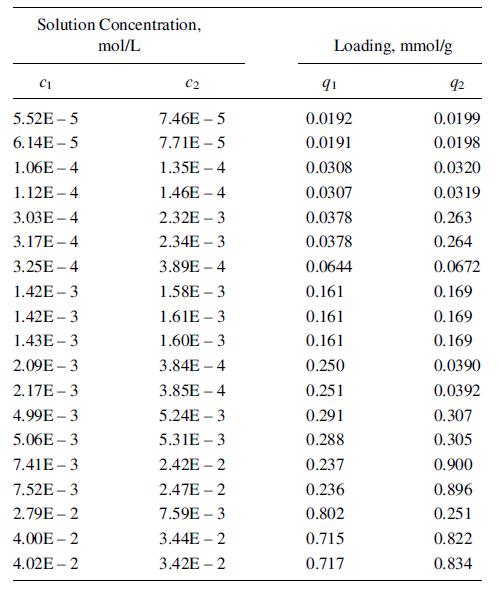
Example 15.6
Small amounts of VOCs in water can be removed by adsorption. Generally, two or more VOCs are present. An aqueous stream containing small amounts of acetone (1) and propionitrile (2) is to be treated with activated carbon. Single-solute equilibrium data from Radke and Prausnitz [37] have been fitted to the Freundlich and Langmuir isotherms, (15-35) and (15-36), with the average deviations indicated, for solute concentrations up to 50 mmol/L:
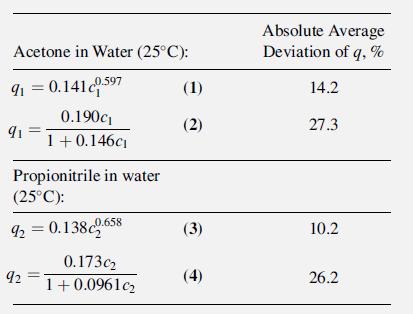
where qi ¼ amount of solute adsorbed, mmol/g, and ci ¼ solute concentration in aqueous solution, mmol/L.
Use these single-solute results with an extended Langmuir-type isotherm to predict the equilibrium adsorption in a binary-solute, aqueous system containing 40 and 34.4 mmol/L, respectively, of acetone and propionitrile at 25C with the same adsorbent. Compare the results with the following experimental values from Radke and Prausnitz [37]:
q1 = 0:715 mmol/g; q2 = 0:822 mmol/g; and qtotal = 1:537 mmol/g
di 1/ni (40) Kic!/mi 1+ ;c 1/n; (1)
Step by Step Solution
3.40 Rating (172 Votes )
There are 3 Steps involved in it
To fit the binary data to the extended LangmuirFreundlich isotherm we need to determine the values of the parameters k1 k2 n1 and n2 in the following ... View full answer

Get step-by-step solutions from verified subject matter experts


