Question: Toluene is hydrodealkylated to benzene, with a conversion per pass through the reactor of 70%. The toluene must be recovered and recycled. Typical conditions for
Toluene is hydrodealkylated to benzene, with a conversion per pass through the reactor of 70%. The toluene must be recovered and recycled. Typical conditions for the feed to a commercial distillation unit are 100οF, 20 psia, 415 lbmol/h of benzene, and 131 lbmol/h of toluene. Using the property constants below, and assuming the ideal-gas, ideal-liquid-solution model of Table 2.4, prove that the mixture is a liquid and estimate vL and pL in American Engineering units.
Property constants for (2-39) and (2-38), with T in K, are:
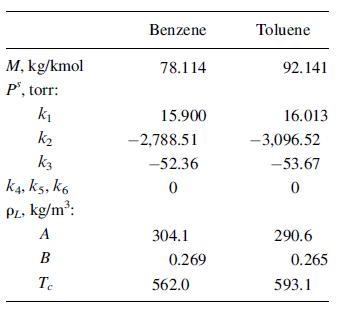
M, kg/kmol P", torr: k k k3 K4, K5, K6 PL, kg/m: A B Te Benzene 78.114 15.900 -2,788.51 -52.36 0 304.1 0.269 562.0 Toluene 92.141 16.013 -3,096.52 -53.67 0 290.6 0.265 593.1
Step by Step Solution
3.52 Rating (159 Votes )
There are 3 Steps involved in it
Proof The chemical reaction is as follows This reaction is second order with respect to benzene firs... View full answer

Get step-by-step solutions from verified subject matter experts


