Question: In a simple cubic perovskite like SrTiO 3 , all oxygens are equivalent. Each makes two bonds to Ti 4+ and four to Sr 2+
In a simple cubic perovskite like SrTiO3, all oxygens are equivalent. Each makes two bonds to Ti4+ and four to Sr2+, as shown in a bond graph below.
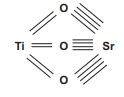
(a) Draw a comparable bond graph for a 1:1 ordered perovskite Ba2ScTaO6 that obeys Pauling’srule of parsimony.
(b) Use the bond graph to estimate the Ba–O, Sc–O, and Ta–O bond valences.
(c) Draw a comparable bond graph for the 1:2 ordered perovskite Ba3ZnTa2O9 and determine the minimum number of chemical environments needed for oxygen is this structure. For each such type of oxygen, determine the number of bonds it makes to each cation.
(d) What are the bond valences for Ba–O, Zn–O, and Ta–O? What are the bond-valence sums for each type of oxygen?
(e) Based on your answers to parts (b) and (d), do the oxygen bond valences suggest a bonding instability that might trigger an SOJT distortion of the Ta5+ ions? Explain your reasoning.
Ti 0 Sr 0
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
a Parsimony rule suggests that all oxygens are equivalent This is possible with each O ... View full answer

Get step-by-step solutions from verified subject matter experts


