In its charged state, a leadacid battery contains PbO 2 and Pb electrodes. The half equations that
Question:
In its charged state, a lead–acid battery contains PbO2 and Pb electrodes. The half equations that occur during discharge can be written as below. Give the overall cell equation and estimate E° cell. State which electrode is the cathode and which the anode.
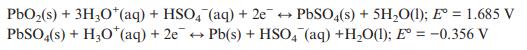
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:





