Perovskites with partially filled e g orbitals at the octahedrally coordinated atom respond to this impending violation
Question:
Perovskites with partially filled eg orbitals at the octahedrally coordinated atom respond to this impending violation of orbital degeneracy in a variety of ways. In SrFeO3, the eg electrons are delocalized and metallic behavior ensues. In LaMnO3, the eg electrons are localized and the octahedra distort through cooperative Jahn–Teller distortions. In CaFeO3, the orbital degeneracy is removed through charge disproportionation into an ordered pattern of Fe3+ and Fe5+. The RNiO3 (R = trivalent rareearth ion) perovskites are another system with partial filling of eg orbitals. The phase diagram between critical temperatures for insulator-to-metal (TIM) and antiferromagnetic-to-paramagnetic (TN) transitions in these nickelates is shown in the figure below (SmNd approximates Pm).
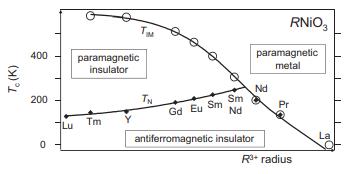
(a) What is the oxidation state, d-electron count and occupation of the t2g and eg orbitals for the low-spin nickel in these compounds?
(b) Would you expect the nickel-centered octahedra in LaNiO3 to be symmetric or to be distorted as a result of a cooperative Jahn–Teller distortion?
(c) Whereas LaNiO3 is a paramagnet and a metallic conductor at all temperatures, the lanthanoid RNiO3 phases undergo a metal–insulator transition upon cooling. What stabilizes metallic LaNiO3 even at low temperatures and why?
(d) Why does the insulator-to-metal transition temperature TIM increase along the rare-earth series?
(e) Which changes in the crystal structure of YNiO3 would you expect upon cooling through TIM? How would the Ni–O distances allow you to see whether the low-temperature paramagnetic insulating phase is stabilized by a cooperative Jahn–Teller distortion or by a charge disproportionation?
Step by Step Answer:

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt





