Question: The phosphor BaMgAl 10 O 17 :Eu 2+ ,Mn 2+ , whose excitation and emission spectra are shown below, is of interest as a combined
The phosphor BaMgAl10O17:Eu2+,Mn2+, whose excitation and emission spectra are shown below, is of interest as a combined blue and green phosphor in plasma-display panels. The Eu2+ ions absorb at 336 nm and emit in the blue with a maximum of 450 nm. There is also energy transfer from Eu2+ to Mn2+ that leads to the green emission at 512 nm.
(a) What are the electronic transitions responsible for emission on Eu2+ and Mn2+?
(b) What is the Stokes shift for Eu2+?
(c) What is the most likely mechanism of energy transfer from Eu2+ to Mn2+, Förster (FRET) or Dexter?
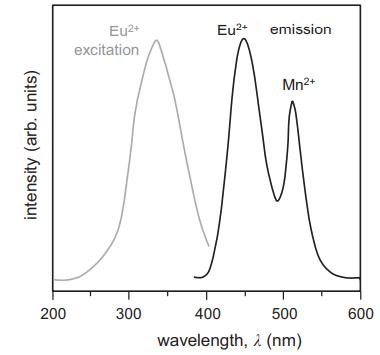
intensity (arb. units) 200 Eu+ excitation 300 Eu+ emission Mn+ 400 500 wavelength, (nm) 600
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
a Eu emits via a Xe4f65d Xe4f transition and Mn emits ... View full answer

Get step-by-step solutions from verified subject matter experts


