Suppose that two identical, neighboring atoms have substantial overlap of one s-orbital and one p-orbital (we can
Question:
Suppose that two identical, neighboring atoms have substantial overlap of one s-orbital and one p-orbital (we can assume, for example, that the p-orbitals in the x-direction overlap, and that the other two p-orbitals of the two atoms, pointing in the y- and z-directions, have very little overlap).
(a) In a mathematical program like Mathematica, construct a 4 × 4 matrix of the LCAO matrix elements: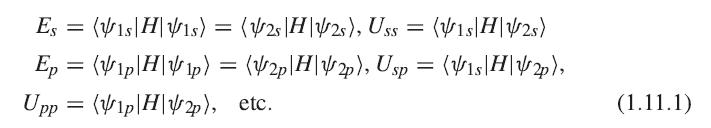
Note that the atomic s- and p-orbitals of a single atom are orthogonal. Assume that the coupling integrals are all real.
(b) Let Es = 1, Ep = 1.3, Upp = 3Uss, and Usp = Uss. Plot the eigenvalues of the matrix as a function of Uss for Uss in the range 0–1. Will this system allow bound states?
(c) Solve for the eigenvectors at Uss = 0.6. What is the character of the states – which are mostly p-like? Which are mostly s-like? Which are bonding? Which are antibonding?
(d) What happens to the character of the bonds if you change the relative weights of the coupling terms Uss, Usp, and Upp? Try plotting the eigenvalues vs Uss for some different values of the ratios.
Step by Step Answer:






