Question: Problem S2.6. A solution of particles A and B has a Gibbs free energy Initially, the solution has n moles of A and ng moles
Problem S2.6. A solution of particles A and B has a Gibbs free energy
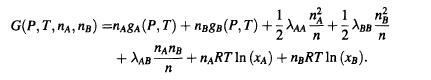
Initially, the solution has n moles of A and ng moles of B.
(a) If an amount, Ang, of B is added keeping the pressure and temperature fixed, what is the change in the chemical potential of A?
(b) For the case AAA = ABB AAB, does the chemical potential of A increase or decrease?
1 n G(P,T, NA, nB)=nAgA (P,T)+nB8B (P,T)+AA- BB nnB n +AB +nRT In (xA) +nBRT ln (xB). n n
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


