S N 2 reactions of simple carboxylate ions with haloalkanes in aqueous solution generally do not give
Question:
SN2 reactions of simple carboxylate ions with haloalkanes in aqueous solution generally do not give good yields of esters.
(a) Explain why this is so.
(b) Reaction of 1-iodobutane with sodium acetate gives an excellent yield of ester if carried out in acetic acid (as shown here). Why is acetic acid a better solvent for this process than water?
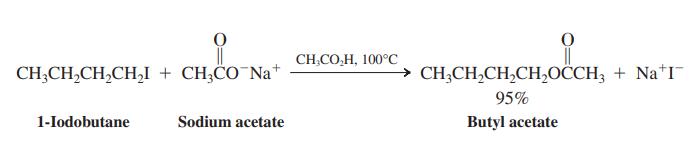
(c) The reaction of 1-iodobutane with sodium dodecanoate proceeds surprisingly well in aqueous solution, much better than the reaction with sodium acetate (see the following equation). Explain this observation.
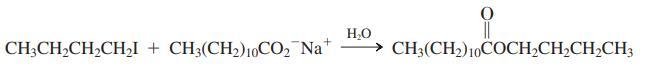
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





