Question: Vanillin, whose structure is shown in the margin and is the subject of the Chapter Opening, is a benzene derivative with several functional groups, each
Vanillin, whose structure is shown in the margin and is the subject of the Chapter Opening, is a benzene derivative with several functional groups, each one of which displays its characteristic reactivity. What would you expect to be the products of reaction of vanillin with each of the following reagents?
(a) NaBH4, CH3CH2OH
(b) NaOH, then CH3I
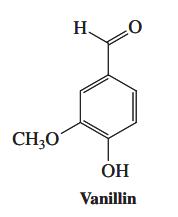 Vanillin is obtained by extraction from the seed pods of plants in the Vanilla genus in a process that dates back at least 500 years: The Mexican Aztecs used it to fl avor xocoatl, a chocolate drink. Cortez discovered it in the court of Montezuma and was responsible for introducing it to Europe. Increasing demand for vanillin necessitated development of synthetic procedures, which involve extraction of related compounds from other plant-derived sources. One of the most important of these sources is the wood-derived waste from the manufacture of paper. The conversion of eugenol (an extract from cloves) into vanillin is very similar chemically. First, treatment of eugenol with KOH at 150 8C in a high-boiling solvent causes positional isomerization of its side-chain double bond:
Vanillin is obtained by extraction from the seed pods of plants in the Vanilla genus in a process that dates back at least 500 years: The Mexican Aztecs used it to fl avor xocoatl, a chocolate drink. Cortez discovered it in the court of Montezuma and was responsible for introducing it to Europe. Increasing demand for vanillin necessitated development of synthetic procedures, which involve extraction of related compounds from other plant-derived sources. One of the most important of these sources is the wood-derived waste from the manufacture of paper. The conversion of eugenol (an extract from cloves) into vanillin is very similar chemically. First, treatment of eugenol with KOH at 150 8C in a high-boiling solvent causes positional isomerization of its side-chain double bond:

Subsequently, oxidative cleavage,completes the synthesis of vanillin. (c) Propose a mechanism for the isomerization depicted in the scheme.
H . CH;0 OH Vanillin
Step by Step Solution
3.44 Rating (154 Votes )
There are 3 Steps involved in it
Mechanism of ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
970_61d57f260c0c7_827489.pdf
180 KBs PDF File
970_61d57f260c0c7_827489.docx
120 KBs Word File


