What is the longest-wavelength electronic transition in each of the following species? Use molecular-orbital designations such as
Question:
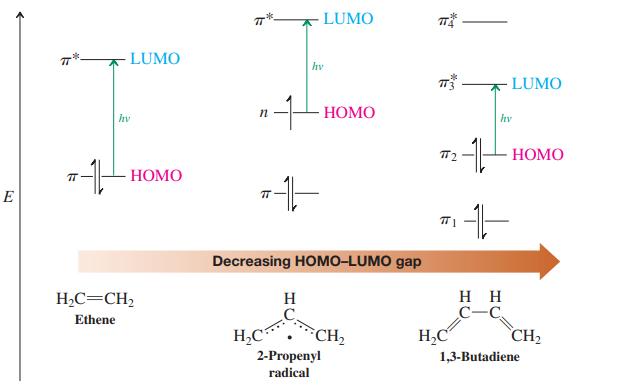
What is the longest-wavelength electronic transition in each of the following species? Use molecular-orbital designations such as n → π*, π1→π2, in your answer. (Prepare a molecular-orbital energy diagram, like that in Figure 14-16, for each.)
(a) 2-Propenyl (allyl) cation;
(b) 2-propenyl (allyl) radical;
(c) formaldehyde, H2C=O;
(d) N2;
(e) pentadienyl anion;
(f) 1,3,5-hexatriene.
Figure 14-16
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





