Question: A rough measure of the enthalpy of combustion of a hydrocarbon can be obtained by assuming that all CC bonds contribute the same energy to
A rough measure of the enthalpy of combustion of a hydrocarbon can be obtained by assuming that all CC bonds contribute the same energy to the combustion process when broken, and that the same is true for all C-H bonds. This is particularly appropriate for alkanes and cycloalkanes, where all C-C bonds are roughly equivalent single bonds. Using this simple model, write a general formula for the combustion energy of an alkane with n carbon atoms and m hydrogen atoms in terms of two variables characterizing the energies of the two bond types. Fit for the two variables by matching with the energy of methane and dodecane, and compare the intermediate energies to those of?
Table 33.1
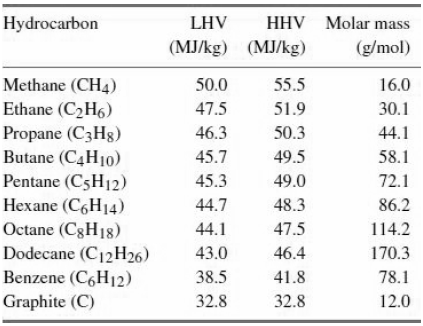
Hydrocarbon LHV HHV Molar mass (MJ/kg) (MJ/kg) (g/mol) Methane (CH4) Ethane (C2H6) Propane (C3H8) Butane (C4H10) Pentane (C5H12) Hexane (C,H14) Octane (C8H18) Dodecane (C12H26) Benzene (C6H12) Graphite (C) 50.0 55.5 16.0 47.5 51.9 30.1 46.3 50.3 44.1 45.7 49.5 58.1 45.3 49.0 72.1 44.7 48.3 86.2 44.1 47.5 114.2 43.0 46.4 170.3 38.5 41.8 78.1 32.8 32.8 12.0
Step by Step Solution
3.41 Rating (170 Votes )
There are 3 Steps involved in it
The number of CH bonds is m and the number of CH b... View full answer

Get step-by-step solutions from verified subject matter experts


