According to Figure 37.7, the energy density of hydrogen compressed to 700 atm is approximately 5.6 MJ/L.Assuming
Question:
According to Figure 37.7,
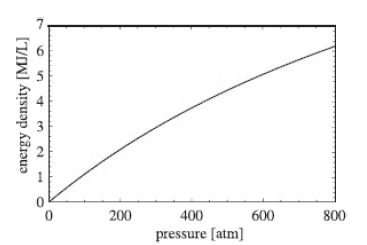
the energy density of hydrogen compressed to 700 atm is approximately 5.6 MJ/L.Assuming hydrogen behaves as an ideal gas throughout,estimate the energy density of hydrogen compressed to 700 atm by adding the enthalpy of combustion and the energy required to compress the hydrogen isothermally starting at 1 atm. How does your answer compare with the quoted figure of 5.6 MJ/L? Compare the total energy stored in hydrogen compressed to 700 atm with the energy density of gasoline.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





