Carbon dioxide sublimes at pressures below roughly 5 atm? At a pressure of 2 atm this phase
Question:
Carbon dioxide sublimes at pressures below roughly 5 atm?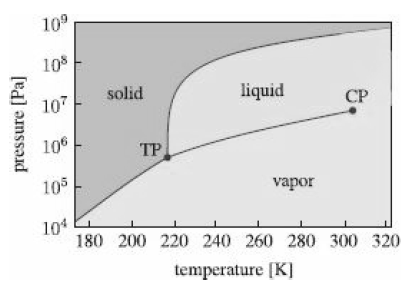
At a pressure of 2 atm this phase transition occurs at about ?69 ?C with an enthalpy of sublimation of roughly 26 kJ/mol. Suppose a kilogram of solid CO2 at a temperature of ?69??C is confined in a cylinder by a piston that exerts a constant pressure of 2 atm. How much heat must be added to completely convert it to gas? Assuming CO2 to be an ideal gas, how much work was done by the CO2 in the course of vaporizing? If the ambient pressure outside the cylinder is 1 atm, how much useful work was done? Finally, by how much did the internal energy of the CO2 change when it vaporized?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





