Calculate the higher and lower heating values of gaseous methane fuel (CH 4 ). Compare your results
Question:
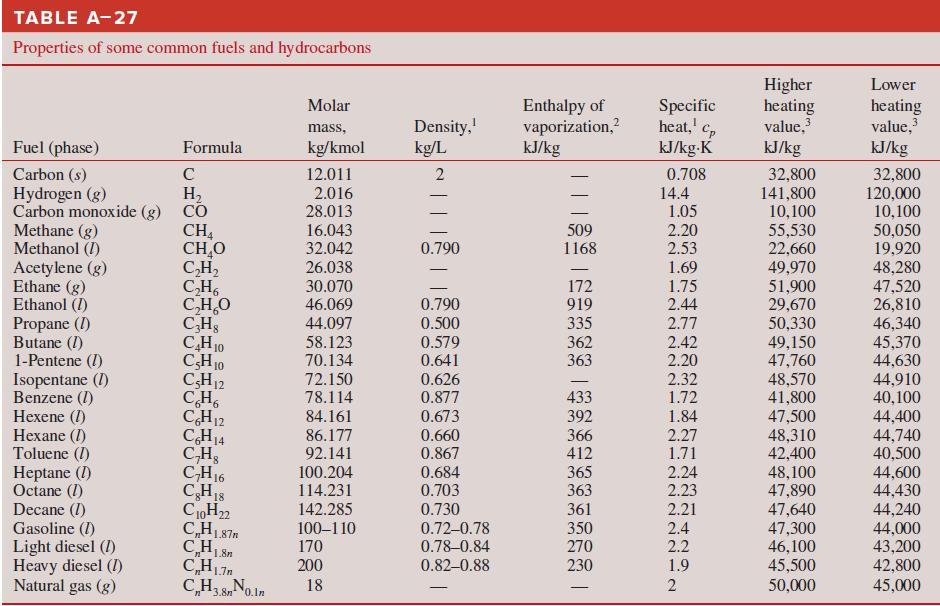
Calculate the higher and lower heating values of gaseous methane fuel (CH4). Compare your results with the values in Table A–27.
Transcribed Image Text:
TABLE A-27 Properties of some common fuels and hydrocarbons Higher heating value, kJ/kg Lower Enthalpy of vaporization,? kJ/kg Molar Density, kg/L Specific heat,' Cp kJ/kg-K heating value, kJ/kg 2 mass, Fuel (phase) Formula kg/kmol Carbon (s) Hydrogen (g) Carbon monoxide (g) CO Methane (g) Methanol (I) Acetylene (g) Ethane (g) Ethanol (I) Propane (I) Butane (I) 1-Pentene (I) Isopentane (1) Benzene (1) Hexene (I) Hexane (I) Toluene (I) Heptane (I) Octane (1) Decane (I) Gasoline (I) Light diesel (I) Heavy diesel (I) Natural gas (g) 32,800 141,800 10,100 55,530 22,660 49,970 51,900 29,670 50,330 49,150 47,760 48,570 41,800 47,500 48,310 42,400 48,100 47,890 47,640 47,300 46,100 45,500 C 12.011 0.708 32,800 120,000 10,100 50,050 19,920 48,280 47,520 26,810 46,340 45,370 44,630 44,910 40,100 H, 2.016 28.013 14.4 1.05 16.043 32.042 26.038 CH, CH,O C,H, C,H, C,H,O CHs CH10 CH10 CH12 CH, CH12 CH14 C,H, CH16 CH1S C0H22 C,HL87n 509 1168 2.20 0.790 2.53 1.69 30.070 46.069 172 919 335 362 363 1.75 0.790 2.44 44.097 0.500 2.77 58.123 70.134 72.150 0.579 0.641 2.42 2.20 2.32 0.626 0.877 433 392 366 412 78.114 1.72 84.161 0.673 1.84 44,400 44,740 40,500 44,600 44,430 44,240 44,000 43,200 42,800 45,000 86.177 0.660 0.867 2.27 1.71 2.24 92.141 100.204 114.231 0.684 0.703 365 363 2.23 142.285 0.730 361 2.21 100–110 170 0.72-0.78 0.78-0.84 350 270 2.4 2.2 200 0.82–0.88 230 1.9 CH3 8No.in 18 50,000 n* *3.8n
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
The higher and lower heating values of gaseous methane are to be determined and compared to the list...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Engineering questions
-
Calculate the higher and lower heating values of a coal from Illinois which has an ultimate analysis (by mass) as 67.40 percent C, 5.31 percent H2, 15.11 percent O2, 1.44 percent N2, 2.36 percent S,...
-
A natural gas is analyzed and found to consist of 85.5% v/v (volume percent) methane, 8.5% ethane, 2.5% propane, and 3.5% N 2 (noncombustible). (a) Calculate the higher and lower heating values of...
-
Calculate the higher and lower heating values of a coal from Utah which has an ultimate analysis (by mass) of 61.40 percent C, 5.79 percent H 2 , 25.31 percent O 2 , 1.09 percent N 2 , 1.41 percent...
-
B sold shares of a qualified small business corporation (QSBC) in the current year realizing a capital gain of $640,000 and shares of a public company realizing a loss of $40,000. B has a net capital...
-
Refer to the table in the margin for n = 8 and p = 0.381. When a car buyer is selected at random, there is a 0.381 probability that he or she bought a used car (based on data from a CAA members'...
-
What type of revenue is reported in the Other revenue section of the multiple-step income statement?
-
What are the two key financial objectives in the management of a company? How can a focus on these objectives create ethical dilemmas?
-
Svedin Incorporated provides the following information relating to 2011: Net income . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . $17,650...
-
K On December 31, 2024, when the market interest rate is 12%, McCann Realty issues $900,000 of 13.25%, 10-year bonds payable. The bonds pay interest semiannually. McCann Realty received $964,699 in...
-
Marcello is a stockbroker who earns $120,000 annually. He owns a disability insurance (DI) policy with an "own occupation" definition of disability that would pay him $6,000 per month in the event of...
-
n-Butane (C 4 H 10 ) is burned with the stoichiometric amount of air in a cook stove. The products of combustion are at 1 atm pressure and 40C. What fraction of the water in these products is liquid?...
-
n-Octane [C 8 H 18 (g)] is burned with the stoichiometric amount of air. Determine the maximum work that can be produced, in kJ/kg fuel, when the air, fuel, and products are all at 25C and 1 atm.
-
Find the limit, if it exists, or show that the limit does not exist. lim (xy 4y)| (, ) (3, 2)
-
Most studies of mutual fund performance conclude that managers cannot consistently exceed the average return in the stock market as a whole. Why might you expect this result? What does it imply about...
-
Explain why the thrombin time is abnormal in patients with afibrinogenemia and dysfibrinogenemia Option. Explain why the prothrombin time but not the APTT is prolonged in FVII deficiency Option....
-
Generous Motors is offering its customers two financing choices on its popular line of Ventura automobiles. Under Option A, customers receive a $1,000 rebate. Under Option B, customers receive a...
-
Your client purchased a home two years ago for $400,000 and put 20% down. The interest rate on the loan was 3.50% with a loan term of 30 years. They have made two years' worth of payments. How much...
-
Look under your bed for dust bunnies. If there arent any, look under your roommates bed, the refrigerator, or any similar place that might have some. Once you find them, blow one toward another....
-
The following situations involve the provision of non-audit services. a. Providing bookkeeping services to a listed entity. The services were preapproved by the audit committee of the company. b....
-
The packaging division of a company having considered several alternative package designs for the company's new product has finally brought down their choices to two designs of which only one has to...
-
A gaseous fuel with a volumetric analysis of 45 percent CH4, 35 percent H2, and 20 percent N2 is burned to completion with 130 percent theoretical air. Determine (a) The air-fuel ratio (b) The...
-
Reconsider Prob. 15-28. Using EES (or other) software, study the effects of varying the percentages of CH4, H2, and N2 making up the fuel and the product gas temperature in the range 5 to 150oC.
-
Methane (CH4) is burned with dry air. The volumetric analysis of the products on a dry basis is 5.20 percent CO2, 0.33 percent CO, 11.24 percent O2, and 83.23 percent N2. Determine (a) The air-fuel...
-
Moxie Inc., a company that produces typewriter replicas, has fixed costs of $20,000 and variable costs of $18 per unit of output. Their expected unit sales is 10,000 units. What is the unit cost of...
-
What if Scenrio May stays as planned but that your supplier tells you that you will not receive any shipments for the rest of the year for the following games: boggle, telestrations, ticket to ride,...
-
enjamin and Nellie Barker have taxable income of $120,000. Several of the items used to calculate their taxable income included W-2 wages of $150,000, and Net Long-Term Capital Losses of $6,000....

Study smarter with the SolutionInn App


