Question: One kilogram of water vapor at 200 kPa fills the 1.1989-m 3 left chamber of a partitioned system shown in Fig. P337. The right chamber
One kilogram of water vapor at 200 kPa fills the 1.1989-m3 left chamber of a partitioned system shown in Fig. P3–37.
The right chamber has twice the volume of the left and is initially evacuated. Determine the pressure of the water after the partition has been removed and enough heat has been transferred so that the temperature of the water is 3°C.
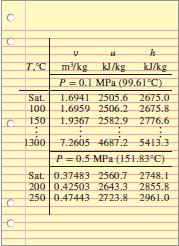
T.C m/kg K/kg P 0.1 MPa (99.61C) KJ/kg Sat. T.6941 2505.6 2675.0 1.6959 2506.2 2675.8 100 150 1.9367 2582.9 2776.6 1300 7.2605 4687.2 5413.3 P=0.5 MPa (151.83C) Sat. 0.37483 2560.7 2748.1 200 0.42503 2643.3 2855.8 250 0.47443 2723.8 2961.0
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
The initial specific volume is At the final state the water occ... View full answer

Get step-by-step solutions from verified subject matter experts


