Question: A polytropic process is one that obeys the relationship PV n = constant, where n may take on various values. A. Define the type of
A polytropic process is one that obeys the relationship PVn = constant, where n may take on various values.
A. Define the type of process associated with each of the following values of n, assuming the working fluid is an ideal gas (i.e., determine which state variable is constant):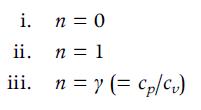
B. Starting from the same initial state, which process results in the most amount of work performed by the gas when the gas is expanded quasi-statically to a final volume twice as large as the initial volume? Which process results in the least amount of work?
C. Sketch the three processes on P–V coordinates.
i. n=0 ii. n = 1 iii. ny (= cp/Cv)
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
A The type of process associated with each of the following values of n assuming the working fluid i... View full answer

Get step-by-step solutions from verified subject matter experts


