Question: Consider the situation described in Problem 7.112. How does the thermal efficiency change if the high-temperature heat-addition process is now conducted at 15MPa instead of
Consider the situation described in Problem 7.112. How does the thermal efficiency change if the high-temperature heat-addition process is now conducted at 15MPa instead of 6 MPa? Be quantitative. How does the net work produced per unit mass of steam (Ẇnet=ṁ) compare for the two cycles?
Problem 7.112
Consider a steady-flow ideal Carnot cycle, using steam as the working fluid, in which the high-temperature, constant-pressure heat-addition process starts with a saturated liquid and ends with a saturated vapor.
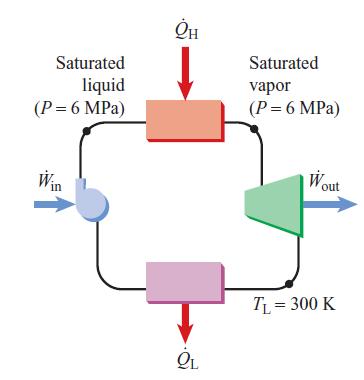
A. Plot this cycle in T–s coordinates showing the steam dome.
B. Calculate the thermal efficiency for this cycle if the pressure of the high temperature steam is 6 MPa and the low-temperature heat-rejection process occurs at 300 K. Calculate the values of the quality at the beginning and end of the heat-rejection process.
C. Explain why it is difficult to operate a practical steam power plant on the ideal Carnot cycle of part A.
Saturated liquid (P = 6 MPa) Win OL Saturated vapor (P = 6 MPa) W out TL = 300 K
Step by Step Solution
3.44 Rating (167 Votes )
There are 3 Steps involved in it
Part A The Carnot cycle in Ts coordinates is shown in the figure below Part B The thermal efficiency ... View full answer

Get step-by-step solutions from verified subject matter experts


