Consider three 0.03-m 3 tanks filled, respectively, with N 2 , Ar, and He. Each tank is
Question:
Consider three 0.03-m3 tanks filled, respectively, with N2, Ar, and He. Each tank is filled to a pressure of 400 kPa at room temperature, 298 K. Determine the mass of gas contained in each tank. Also determine the number of moles of gas (kmol) in each tank.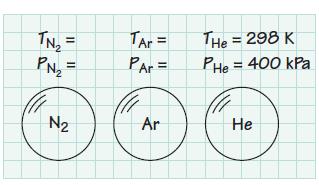
Transcribed Image Text:
= PN₂ = 3 N₂ TAr = PAr = Ar THE = 298 K Phe = 400 kPa He
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To determine the mass and number of moles of gas in each tank we need to use the ideal gas law which ...View the full answer

Answered By

Saleem Abbas
Have worked in academic writing for an a years as my part-time job.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
A 3 m3 tank initially contains air at 100 kPa and 25oC. The tank is connected to a supply line at 550 kPa and 25oC. The valve is opened, and air is allowed to enter the tank until the pressure in the...
-
Consider a 2.00-mole sample of Ar at 2.00 atm and 298 K. a. If the gas sample expands adiabatically and reversibly to a pressure of 1.00 atm, calculate the final temperature of the gas sample...
-
On January 1, 2020, Brown Mining Enterprises purchased an existing coalmine. Brown expects to operate the mine for four years (i.e., until the end of 2023), after which it is legally required to...
-
Antonio Banderos & Scarves makes headwear that is very popular in the fall/winter season. Units sold are anticipated as: October .......... 1,250 November ......... 2,250 December ......... 4,500...
-
At the beginning of 2016, Better Corp.s accounting records had the following general ledger accounts and balances. Better Corp. completed the following transactions during 2016: 1. Purchased land for...
-
Which of the following statements about Treasury bills is false? a. T-bills sell at a discount from face value and pay the face value at maturity. b. T-bills have maturities of 2, 3, 5, 7, or 10...
-
An airplane flies at \(150 \mathrm{~km} / \mathrm{hr}\). (a) The airplane is towing a banner that is \(b=0.8 \mathrm{~m}\) tall and \(\ell=25 \mathrm{~m}\) long. If the drag coefficient based on area...
-
The advertising director a large retail store in Columbus, Ohio, is considering three advertising media possibilities: (1) ads in the Sunday Columbus Dispatch newspaper, (2) ads in a local trade...
-
Problem #4: Air at 100 kPa and 300 K flows steadily through a square duct with side length of 0.5 m. The air enters with a uniform speed of 5 m/s. An electric surface heating element is attached to...
-
Branson Co. received its bank statement for the month ending May 31, 2022, and reconciled the statement balance to the May 31, 2022, balance in the Cash account. The reconciled balance was determined...
-
A pistoncylinder arrangement in an air separation plant contains nitrogen at 21 C and 1.379 MPa. The piston moves and compresses the nitrogen from 98 to 82 cm 3 . The final (equilibrium) temperature...
-
Use Table C.2 to calculate the mass-specific enthalpy change for air undergoing a change of state from 300 K to 1000 K. How does this value compare with that estimated using the constant-pressure...
-
Explain the differences among creativity, innovation, and entrepreneurship.
-
1) The conducted emission test limit for a class A digital device is 79 dBV at 150 kHz for the voltage measured across a 50 resistor. Find the limit in A. Round the result to the nearest integer 1...
-
Option ExplicitSub generateNewProblem ( ) ' It generates a new case for knapsack problem ' It uses random numbers for weights, values and maximum weight Dim W As Long Dim n As Long Dim i As Long Dim...
-
Do you think it is right for organizations to set up organizational display rules? (1) state your position (Yes or No) and (2) give 3 reasons to justify your position. (Hint: Emotional labor)
-
A city has just added 100 new female recruits to its police force. The city will provide a pension to each new hire who remains with the force until retirement. In addition, if the new hire is...
-
An analyst is bullish about the performance of Company X, after reviewing their latest product. He is offered the following two options to invest: Portfolio 1: Buy 100 units of ATM call options with...
-
The table shows the political affiliations of U.S. voters and their positions on supporting stronger immigration enforcement. a) What's the probability that i) A randomly chosen voter favors stronger...
-
Explain the term "Equivalent Units". Why are they calculated in process costing? [4 Marks] [minimum 350 words]
-
A cycloid is the curve described by a point P on the circumference of a circular wheel of radius r rolling along the x axis. The curve is described in parametric form by the equations Use these...
-
Afence around a eld is shaped as shown in Figure P25. It consists of a rectangle of length L and width W and a right triangle that is symmetric about the central horizontal axis of the rectangle....
-
The four-sided gure shown in Figure P26 consists of two triangles having a common side a. The law of cosines for the top triangle states that a 2 = b 2 1 + c 2 1 - 2b 1 c 1 cos A 1 and a similar...
-
2. [50 points] The problem of transient radial heat flow in a circular rod in nondimensional form is described by Ju -2 1 = It with boundary conditions u(t, 1) = 1 and (t, 0) = 0 Or u(0,r)=0 0
-
Consider the system shown below, where gain K is any real number and P(s): r K P(s) y 35 2 1 = S+4 (a) First find A(s) and then use it to compute the characteristic polynomial. [2 marks] (b) Suppose...
-
A)Calculate the clean stall speed (Vs) of the following aircraft (2 pts) Wing area: 174 sq ft CL max zero flaps: 1.22 CL max full flaps: 1.7 Weight: 2,550 lbs Standard density of 0.002377 ...

Study smarter with the SolutionInn App


