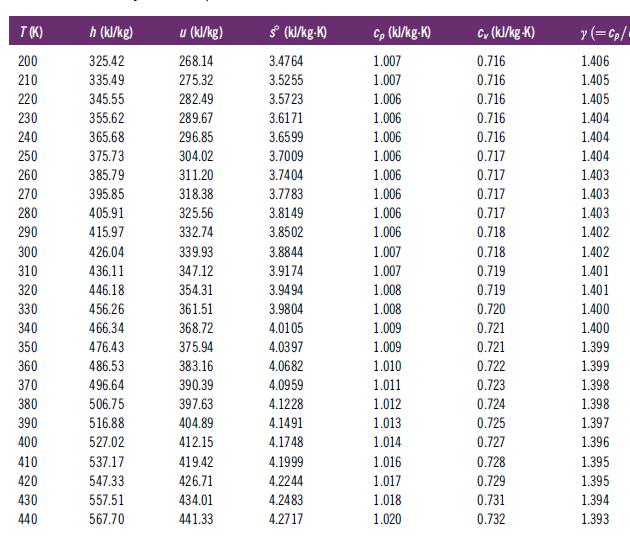
Determine the enthalpy change for air undergoing a process that causes a change of state from 300
Question:
Determine the enthalpy change for air undergoing a process that causes a change of state from 300 K and 1 atm to 1000 K and 5 atms. Do this in two ways: (1) Assume ideal gas behavior and use Table C.2, and (2) use the NIST software, which incorporates real gas behavior. How do the two results compare? Why do the results differ? One objective of this problem is to acquaint you with ways to find the calorific properties of air.
Table C.2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:





