For temperatures between 300 and 1000 K and at 1 atm, the molar specific enthalpy of O
Question:
For temperatures between 300 and 1000 K and at 1 atm, the molar specific enthalpy of O2 is expressed by the following polynomial: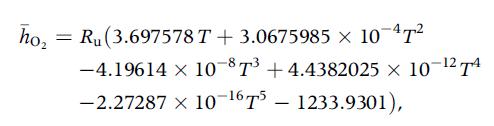
where h̅ is expressed in kJ/kmol and T in kelvins.
Determine the constant-pressure molar-specific heat c̅p at 500 K and at 1000 K. Compare the magnitudes of the values at the two temperatures and discuss your results. Also determine the constant-pressure mass-specific heat cp.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:





