In Problem 7.94, there are two ways to calculate the change in entropy of the system during
Question:
In Problem 7.94, there are two ways to calculate the change in entropy of the system during this reversible process. Find the entropy of the system using both of these methods.
Problem 7.94
The air in a piston–cylinder device initially has a volume of 0.2 m3 at 100 kPa and 200° C. The air is compressed reversibly from 100 kPa to 300 kPa at a constant temperature of 200° C. The air temperature in the cylinder is maintained constant during this process as a result of heat transfer to the surrounding medium at 80° C.
A. Calculate the P–V work during the isothermal process and use an energy balance to find the heat transfer from the air in the cylinder. Then calculate the change in entropy for the reversible isothermal heat transfer process, using Eq. 7.2a.
Eq. 7.2a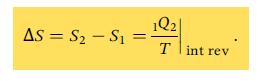
B. Find the mass of air in the cylinder and use Eq. 7.12a to find the change in entropy of the air.
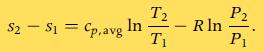
C. Do these two methods give the same answer?
D. Review Section 7.1e, “Gibbs Relationships”, and explain why the two methods give the same answer for the change in entropy.
Eq7.12b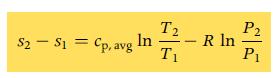
Step by Step Answer:

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley





