Table 41.3 shows that for the ground state of the potassium atom, the outermost electron is in
Question:
Table 41.3
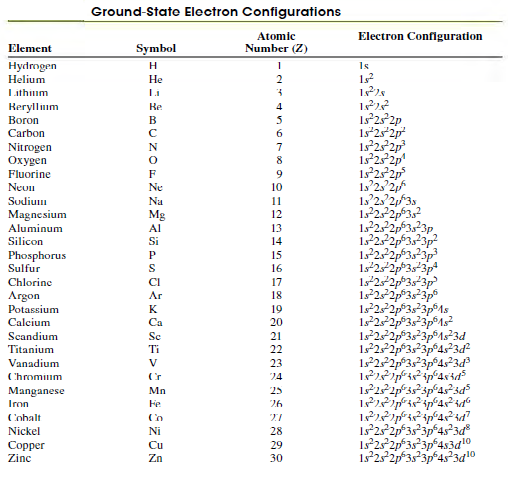
Transcribed Image Text:
Ground-State Electron Configurations Electron Configuration Atomic Element Symbol Number (Z) Hydmgen Helium н Is 1s2 Не Lithium Reryllum Boron Re 4 Is°25°2p 1s°252p 1s 25 2p 125 2p Carbon 6. Nitrogen Охудen Fluorine 9. 1s'23'2, 1s 2. 2,f3s 1522p3,? 12,2323p Neun Ne 10 Sudium Na 11 Magnesium Mg 12 Aluminum 13 Silicon Si 14 152,2p323p 1s*22p*3s*3p* 1s22p*3s*3p 1s22p®3s²3p® Phosphorus Sulfur 15 16 Chlorine CI 17 Argon Ar 18 Potassium K 19 1s252p®3s²3p®1s° 1s252p®3s²3p®1s°3d 122p°3,²3p°4s°3d 122p°3s°3p®4s°3d Calcium Ca 20 Scandium Sc 21 Titanium Ti 22 Vanadium 23 Chromium 24 1s25 ip 3s sp°4s 3d Manganese Iron Mn 25 Fe 26 Cobalt Is°2s°2p*3s3p*4s°3d Is252p35 3p*4s3d10 1s252p*3s 3p 45 3d10 Nickel Ni 28 Copper Zinc Cu 29 Zn 30
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (17 reviews)
The 4s level lies be...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

University Physics with Modern Physics
ISBN: 978-0133977981
14th edition
Authors: Hugh D. Young, Roger A. Freedman
Question Posted:
Students also viewed these Physics questions
-
Write an orbital diagram for the ground state of the potassium atom. Is the atomic substance diamagnetic or paramagnetic?
-
Using Table 22.3, which lists the possible terms that arise from a given configuration, and Hunds rules, write the term symbols for the ground state of the atoms K through Cu, excluding Cr, in the...
-
BirgeSponer plots are generally made using G for n values far from the dissociation limit. If the data include n values close to the dissociation limit, deviations from a linear relationship between...
-
Harvold Company's quality cost report is to be based on the following data: Test and inspection of incoming materials. $71,000 Supplies used in testing and inspection . Re-entering data because of...
-
(a) A mutchkin is a Scottish unit of liquid measure equal to 0.42 L. How many mutchkins are required to fill a container that measures one foot on a side? (b) A noggin is a volume equal to 0.28...
-
Make a copy of the isothermal transformation diagram for a 0.45 wt% C iron-carbon alloy (Figure 10.39), and then sketch and label on this diagram the time-temperature paths to produce the following...
-
Use technology to find the regression line to predict $Y$ from $X$. $X$ 2 4 6 8 10 12 $Y$ 50 58 55 61 69 68
-
A two-evaporator compression refrigeration system as shown in Fig. P11-60 uses refrigerant-134a as the working fluid. The system operates evaporator 1 at 08C, evaporator 2 at 226.48C, and the...
-
here are 4 common categories of customers: new, existing, exiting, and exited. Suppose you work for a large financial institution that is looking to improve its savings deposits and retirement...
-
Access the IOSCO website (www. IOSCO.org), click the About IOSCO section, click the most recent annual report, go to the table of contents and list the working committees.
-
In Example 43.9 (Section 43.4), the activity of atmospheric carbon before 1900 was given. Discuss why this activity may have changed since 1900.
-
For a body orbiting the sun, such as a planet, comet, or asteroid, is there any restriction on the z-component of its orbital angular momentum such as there is with the z-component of the electrons...
-
For a diatomic molecule find the contribution of the vibration energy to the specific heat under constant volume. Neglect the ground level energy effect to the partition function.
-
Consider a positively charged wire bent into the shape of a capital U. Point P is down in the well of the "U" (specifically at th center of curvature). At point P, the direction of the E-field is...
-
What is the acceleration ( in m / s 2 ) needed to stop a 1 5 . 8 kg bike from 2 . 6 km / h to rest in 2 1 . 2 seconds?
-
How does Mary Rowlandson describe the attack that led to her capture? How was Rowlandson treated during her captivity? Why did Rowlandson write her narrative? What might her audience at the time have...
-
how did culture play a big part in their everyday lifestyles and how can you differentiate culture to tradition?
-
(a) Below is an estimate of all her expenses and sources of funding for her 2-year college education. For each, select whether it is an expense or a source of funding. Scholarships: Tuition and fees:...
-
Why is identification of risks, through a listing of assets and their vulnerabilities, so important to the risk management process?
-
The following T-accounts show postings of selected transactions. Indicate the journal used in recording each of these postings a through e. Cash Accounts Receivable Inventory (d) 500 (e) 300 (b)...
-
Let S = 100 mm 2 , d = 3 mm, and r = 12 for a parallel-plate capacitor. (a) Calculate the capacitance. (b) After connecting a 6-V battery across the capacitor, calculate E, D, Q, and the total...
-
Consider a coaxial capacitor having inner radius a, outer radius b, unit length, and filled with a material with dielectric constant, r. Compare this to a parallel-plate capacitor having plate width...
-
The surface x = 0 separates two perfect dielectrics. For x > 0, let r = r 1 = 3, while r 2 = 5 where x < 0. If E 1 = 80a x 60a y 30a z V/m, find (a) EN 1 ; (b) ET 1 ; (c) E 1 ; (d) The angle 1...
-
1. Refer to the graph provided. Price, cost of unit $15- 9 MC ATC MR = P = D a. At what level of output does the firm maximize profit? Explain how you know. b. At the profit-maximizing quantity of...
-
A bond issued 10 years ago had a face value of $2,000; a coupon rate of 5%; and a yield of 6% when it was sold last month in the secondary bond market. At what price did the bond sell in the...
-
What are the assertions affected by the earlier list on what could go wrong in the post to the general journal process? The assertions to use are Completeness Existence/Occurrence Presentation and...

Study smarter with the SolutionInn App


