Question: The following pKa values have been measured. Explain why a hydroxyl group in the para position decreases the acidity while a hydroxyl group in the
The following pKa values have been measured. Explain why a hydroxyl group in the para position decreases the acidity while a hydroxyl group in the Meta position increases the acidity.
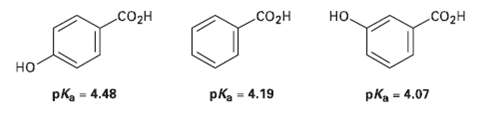
. CO2H CO2H CO2H pka = 4.19 pKa = 4.07 %3D pka - 4.48
Step by Step Solution
3.34 Rating (166 Votes )
There are 3 Steps involved in it
As we have seen throughout this book the influence of sub... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-CA (85).docx
120 KBs Word File


