Question: The following reactivity order has been found for the basic hydrolysis of p-substituted methyl benzoates: Y = NO 2 > Br > H > CH
The following reactivity order has been found for the basic hydrolysis of p-substituted methyl benzoates: Y = NO2 > Br > H > CH3 > OCH3. How can you explain this reactivity order? Where would you expect Y = C ? N = Y = CHO, and Y = NH2 to he in the reactivity list?
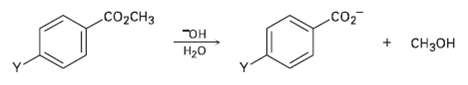
co 2CH D CH H20
Step by Step Solution
3.38 Rating (167 Votes )
There are 3 Steps involved in it
A negatively charged tetrahedral intermediate is formed when the nucleoph... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-CA (140).docx
120 KBs Word File


