The Redlich-Kwong equation of state is given by Where R = the universal gas constant [= 0.518
Question:
The Redlich-Kwong equation of state is given by
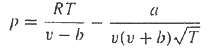
Where R = the universal gas constant [= 0.518 kj/(kg K)], T = absolute temperature (K), p = pressure (kPa), and ?? = the volume of a kg of gas (m3/kg). The parameters a and b are calculated by
Where pc = critical pressure (kPa) and Tc = critical temperature (K). As a chemical engineer, you are asked to determine fuel (pc = 4580kPa and Tc = 191 K) that can be held in a 3-m3 tank at a temperature of -50o C with a pressure of 65,000 kPa. Use a root-locating method of your choice to calculate ?? and then determine the mass of methane contained in thetank.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Numerical Methods For Engineers
ISBN: 9780071244299
5th Edition
Authors: Steven C. Chapra, Raymond P. Canale
Question Posted:





