To enhance the effective surface, and hence the chemical reaction rate, catalytic surfaces often take the form
Question:
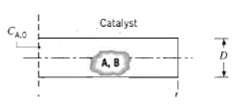
To enhance the effective surface, and hence the chemical reaction rate, catalytic surfaces often take the form of porous solids. One such solid may be visualized as consisting of a large number of cylindrical pores, each of diameter D and length L. Consider conditions involving a gaseous mixture of A and B for which species A is chemically consumed at the catalytic surface. The reaction is known to be first order, and the rate at which it occurs per unit area of the surface may be expressed as k''1 CA, where k''1 (m/s) is the reaction rate constant and CA (k mol/m3) is the local molar concentration of species A. Under steady-state conditions, flow over the porous solid is known to maintain a fixed value of the molar concentration CA.0 at the pore mouth. Beginning from fundamentals obtain the differential equation that governs the variation of CA with distance x along the pore. Applying appropriate boundary conditions solve the equation to obtain an expression for CA(x).
Step by Step Answer:

Fundamentals of Heat and Mass Transfer
ISBN: 978-0471457282
6th Edition
Authors: Incropera, Dewitt, Bergman, Lavine





