Question: Using Table 8.4, select possible liquid liquid extraction solvents for separating the following mixtures: (a) Waterethyl alcohol, (b) Wateraniline, and (c) Wateracetic acid. For each
Using Table 8.4, select possible liquid ”liquid extraction solvents for separating the following mixtures:
(a) Water”ethyl alcohol,
(b) Water”aniline, and
(c) Water”acetic acid. For each case, indicate clearly which of the two components should be the solute.
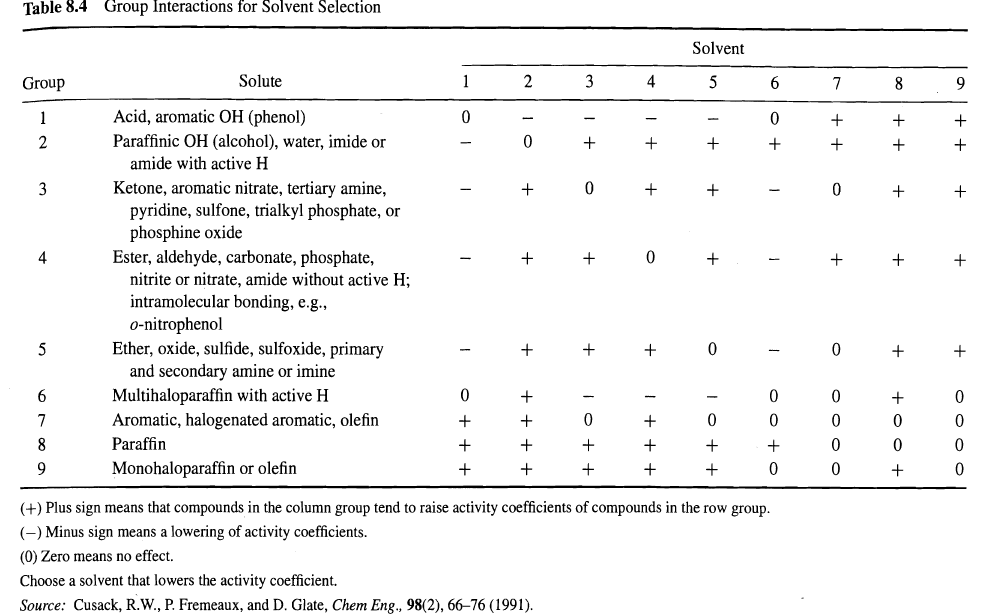
Table 8.4 Solvent Solute 3 4 Group 6. Acid, aromatic OH (phenol) Paraffinic OH (alcohol), water, imide or amide with active H Ketone, aromatic nitrate, tertiary amine, pyridine, sulfone, trialkyl phosphate, or phosphine oxide Ester, aldehyde, carbonate, phosphate, nitrite or nitrate, amide without active H; intramolecular bonding, e.g., 3 4 o-nitrophenol Ether, oxide, sulfide, sulfoxide, primary and secondary amine or imine Multihaloparaffin with active H Aromatic, halogenated aromatic, olefin 6. Paraffin Monohaloparaffin or olefin (+) Plus sign means that compounds in the column group tend to raise activity coefficients of compounds in the row group. (-) Minus sign means a lowering of activity coefficients. (0) Zero means no effect. Choose a solvent that lowers the activity coefficient. Source: Cusack, R.W., P. Fremeaux, and D. Glate, Chem Eng., 98(2), 6676 (1991).
Step by Step Solution
3.48 Rating (164 Votes )
There are 3 Steps involved in it
Subject Selection of extraction solvents Given The following mixtures ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (305).docx
120 KBs Word File


