Question: We noted in Section 12.5 (Figure) that in FeO (wstite), the iron ions can exist in both Fe2+ and Fe3+ states. The number of each
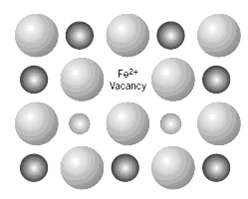
We noted in Section 12.5 (Figure) that in FeO (wüstite), the iron ions can exist in both Fe2+ and Fe3+ states. The number of each of these ion types depends on temperature and the ambient oxygen pressure. Furthermore, we also noted that in order to retain electroneutrality, one Fe2+ vacancy will be created for every two Fe3+ ions that are formed; consequently, in order to reflect the existence of these vacancies the formula for wüstite is often represented as Fe(1 ??? x) O where x is some small fraction less than unity.
In this nonstoichiometric Fe(1 ??? x) O material, conduction is electronic, and, in fact, it behaves as a p-type semiconductor. That is, the Fe3+ ions act as electron acceptors, and it is relatively easy to excite an electron from the valence band into an Fe3+ acceptor state, with the formation of a hole. Determine the electrical conductivity of a specimen of wüstite that has a hole mobility of 1.0 ?? 10???5 m2/V-s and for which the value of x is 0.060. Assume that the acceptor states are saturated (i.e., one hole exists for every Fe3+ ion). Wüstite has the sodium chloride crystal structure with a unit cell edge length of 0.437nm.
Fer+ Vacancy
Step by Step Solution
3.39 Rating (171 Votes )
There are 3 Steps involved in it
We are asked in this problem to determine the electrical conductivity for the nonstoichiometric Fe 1 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (720).docx
120 KBs Word File


