Question: When an unsymmetrical alkene such as propene is treated with N-Bromosuccinimide in aqueous dimethyl sulfoxide, the major product has the bromine atom bonded to the
When an unsymmetrical alkene such as propene is treated with N-Bromosuccinimide in aqueous dimethyl sulfoxide, the major product has the bromine atom bonded to the less highly substituted carbon atom. Is this Markovnikov or non-Markovnikov orientation? Explain.
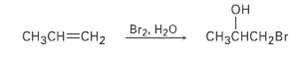
Br2, H20 CH3CH=CH2 CHCH2Br
Step by Step Solution
★★★★★
3.23 Rating (175 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Reaction of the alkene with Br2 formed from NBS produces a cyclic bromonium ion When ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock
Document Format (1 attachment)
22-C-OC-A (142).docx
120 KBs Word File


