Question: You are checking the performance of a reactor in which acetylene is produced from methane in the reaction 2 CH 4 (g) ? C 2
You are checking the performance of a reactor in which acetylene is produced from methane in the reaction 2 CH4 (g) ? C2H2 (g) + 3 H2 (g) an undesired side reaction is the decomposition of acetylene: C2H2 (g) ? 2 C (s) + H2 (g) methane is fed to the reactor at 1500CC at a rate of 10.0 mol CH4/s. Heat is transferred to the reactor at a rate of 975 kW. The product temperature is 1500?C and the fractional conversion of methane is 0.600. A flowchart of the process and an enthalpy table are shown below.
(a) Using the heat capacities given below for enthalpy calculations, write and solve material balances and an energy balance to determine the product component flow rates and the yield of acetylene (mol C2H2 produced/mo! CH4 consumed).
CH4 (g): ? ? ? ? ??Cp?? 0.079 kJ/(mol??C)
C2H2 (g) ? ? ? ? ?Cp?? 0.052 kJ/(mol??C)
H2 (g): ?? ? ? ? ? ??Cp?? 0.031 kJ/(mol??C)
C (s):? ?? ? ? ? ? ??Cp?? 0.022 kJ/(mol??C)
For example, the specific enthalpy of methane at 1500?C relative to methane at 25?C is [0.079 kJ/ (mol??C)] (1500?C ?25?C) = 116.5 kJ/mol.
(b) The reactor efficiency may be defined as the ratio (actual acetylene yield/acetylene yield with no side reaction). What is the reactor efficiency for this process?
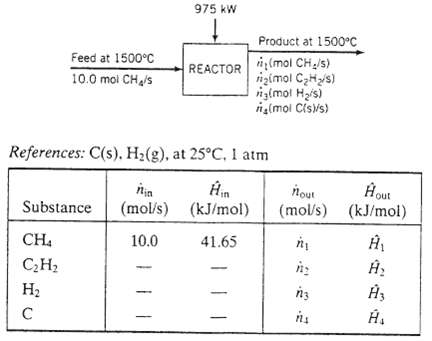
Feed at 1500C 10.0 mol CH/s CH CH H 975 kW Product at 1500C REACTOR (mol CH/s) gimol CH/s) (mol H/s) (mol C(s)/s) References: C(s). H(g), at 25C, 1 atm nin Substance (mol/s) (kJ/mol) 10.0 41.65 nout (mol/s) n n n3 n out (kJ/mol)
Step by Step Solution
3.35 Rating (161 Votes )
There are 3 Steps involved in it
a b 2CH CH 3H CH 2Cs H Basis 100 mol CHgs 1500 C 60 conversion n 1010600 400 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (541).docx
120 KBs Word File


