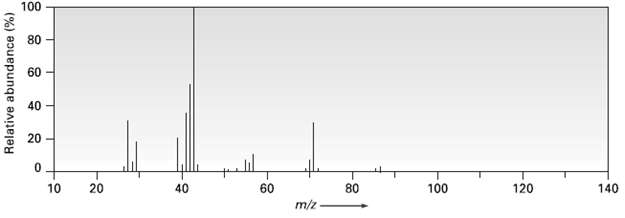
Question: 2-Methylpentanc (C 6 H 14 ) has the mass spectrum shown. Which peak represents M + ? Which is the base peak? Propose structures for
2-Methylpentanc (C6H14) has the mass spectrum shown. Which peak represents M+? Which is the base peak? Propose structures for fragment ions of m/z = 71, 57, 43, and 29. Why does the base peak have the mass it does?
100 80 20 120 40 10 140 20 60 80 100 m/z Relative abundance (%) 20 8
Step by Step Solution
3.37 Rating (153 Votes )
There are 3 Steps involved in it
CHCHCHCHCH mz 71 CH3 CH3CHCHCHCH3 2Methylpentane k ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-S (149).docx
120 KBs Word File


