Question: A 0.530 kg sample of liquid water and a sample of ice are placed in a thermally insulated container. The container also contains a device
A 0.530 kg sample of liquid water and a sample of ice are placed in a thermally insulated container. The container also contains a device that transfers energy as heat from the liquid water to the ice at a constant rate P, until thermal equilibrium is reached. The temperatures T of the liquid water and the ice are given in Figure as functions of time t; the horizontal scale is set by ts = 80.0 min.(a) What is rate P?(b) What is the initial mass of the ice in the container?(c) When thermal equilibrium is reached, what is the mass of the ice produced in thisprocess?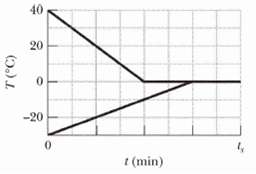
40 20 -20 t (min) T (CC)
Step by Step Solution
3.35 Rating (182 Votes )
There are 3 Steps involved in it
a Eq 1814 in absolute value gives Q 4190 J kg C 0530 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
2-P-T-T (341).docx
120 KBs Word File


