(a) A bottle of pure n-heptane is accidentally poured into a of pure toluene in a commercial...
Question:
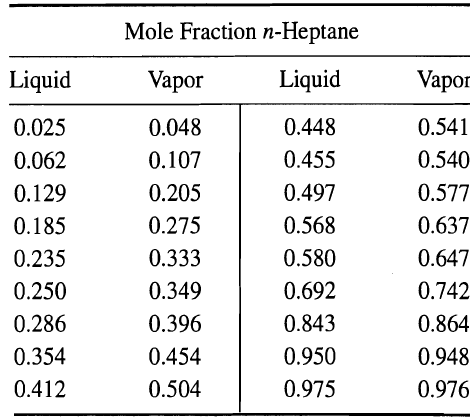
(a) A bottle of pure n-heptane is accidentally poured into a of pure toluene in a commercial laboratory. One of the laboratory assistants, with almost no background in chemistry, suggests that, since heptane boils at a lower temperature than toluene, the following purification procedure can be used: Pour the mixture (2 mol% n-heptane) into a simple still pot. Boil the mixture at 1 atm and condense the vapors until all heptane boiled away. Obtain the pure toluene from the residue in the still pot. You, being a chemical engineer, immediately realize that such a purification method will not work. Indicate this by a curve showing the composition of the material remaining m the pot after various quantities of the liquid have been distilled. What is the composition of the residue after 50 wt% of the original material has been distilled? What is the composition of the cumulative distillate?
(b) When one-half of the heptane has been distilled, what is the composition of the cumulative distillate and of the residue? What weight percent of the original material has been distilled? Vapor'liquid equilibrium data at 1 atm (Ind. Eng. Chem., 42, 2912 (1949)1 are asfollows:
Step by Step Answer:






