A Alkynes can be made by dehydrohalogenation of vinylic halides in a reaction that is essentially an
Question:
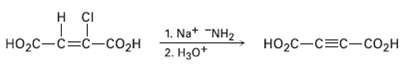
A Alkynes can be made by dehydrohalogenation of vinylic halides in a reaction that is essentially an E2 process. In studying the stereochemistry of this elimination, it was found that (Z)-2-chloro-2-butenedioic acid reacts 50 times as fast as the corresponding E isomer. What conclusion can you draw about the stereochemistry of eliminations in vinylic halides? How does this result compare with eliminations of alkylhalides?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





