Question: (a) Calculate the work W L that would be required to liquefy one mole of a monatomic ideal gas if the liquefier operated reversibly. Assume
(a) Calculate the work WL that would be required to liquefy one mole of a monatomic ideal gas if the liquefier operated reversibly. Assume that the gas is supplied at room temperature To, and tinder the same pressure po at which the liquefied gas is removed, typically I atmosphere, Let Tb be the boiling temperature of the as at this pressure and ?H the latent heat of vaporization. Show that under these conditions
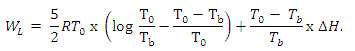
To derive (11) assume that the gas is first cooled at fixed pressure p0 from T0 to Tb, by means of a reversible refrigerator that operates between the fixed upper temperature Th = T0 and a variable lower temperature equal to the gas temperature. Initially T1 = T0. and at the end Tt = Tb. After reaching Tb the refrigerator extracts the latent heat of vaporization at the fixed lower temperature Tb.
(b) Insert T0 = 300K and values for Tb and ?H characteristic of helium. Re-express the result as kilowatt-hours per liter of liquid helium. Actual helium liquefiers consume S to 10kWh, liter.
. - . - (log To _T- , To 3RT, (1og . W. %3D
Step by Step Solution
3.39 Rating (168 Votes )
There are 3 Steps involved in it
a b The reversible work dw required to cool the id... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
42-P-S-S-T-T (77).docx
120 KBs Word File


