Question: (a) Estimate the liquefaction coefficient for helium by treating it as a van der Waals gas. Select the van der Waals coefficients a and
(a) Estimate the liquefaction coefficient λ for helium by treating it as a van der Waals gas. Select the van der Waals coefficients a and b in such a way that fur one mole 2Wb is the actual molar volume of liquid helium and that 2u/b is the actual inversion temperature Use the data in Table 12.1. Approximate the denominator in (7) by settling
Hout – Htiq ≈ ∆h + 5/2 (τin – τtiq),
where ∆H is the latent heat of vaporization of liquid helium. (Explain how this approximation arises if on treats the expanded gas as an ideal). The resulting expression gives λ a function of the molar volumes Vin and Vout. Convert to pressures by approximating the Vs via the ideal gas law,
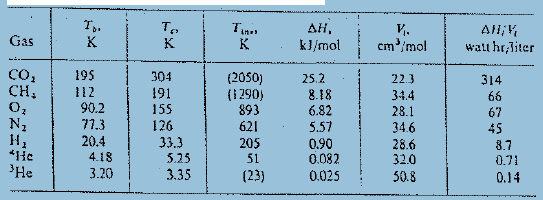
(b) Insert numerical values for T = 15 K and compare with figure.
, k}/mol . cm/mol Gas All,V watt hr,liter K. CO, CH, 304 191 155 126 33.3 5.25 3.35 195 (2050) (i 290) 893 621 205 51 (23) 25.2 8.18 6.82 5.57 0.90 0.082 0.025 314 22.3 34.4 28.1 34.6 28.6 12 90.2 77.3 20.4 4.18 3.20 66 67 N1 45 8.7 , *He 32.0 0.71 'He 50.8 0.14
Step by Step Solution
3.39 Rating (171 Votes )
There are 3 Steps involved in it
a b van der Waals gas If we set Nb We use the approximation 4 for the enthalpy ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
42-P-S-S-T-T (76).docx
120 KBs Word File


