Question: Tritium ( ) is a rare isotope of hydrogen that can be produced by the following fusion reaction: (a) Determine the atomic mass number A,
Tritium (![]() ) is a rare isotope of hydrogen that can be produced by the following fusion reaction:
) is a rare isotope of hydrogen that can be produced by the following fusion reaction:
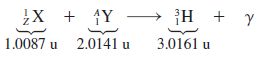
(a) Determine the atomic mass number A, the atomic number Z, and the names X and Y of the unknown particles.
(b) Using the masses given in the reaction, determine how much energy (in MeV) is released by this reaction.
.0087 2.0141 3.0161 u
Step by Step Solution
3.27 Rating (162 Votes )
There are 3 Steps involved in it
The conservation of nucleon number states that the total number of nucleons protons plus neutrons be... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
1176-P-M-P-A-S(1691).docx
120 KBs Word File


