Find the energy emitted in the decay of 239 Pu. Strategy Nuclear reaction energy, such as
Question:
Find the energy emitted in the α decay of 239Pu.
Strategy
Nuclear reaction energy, such as released in a decay, can be found using the equation E =(Δm)c2. We must first find Δm, the difference in mass between the parent nucleus and the products of the decay. This is easily done using masses given in Appendix A.
Data from Appendix A
Transcribed Image Text:
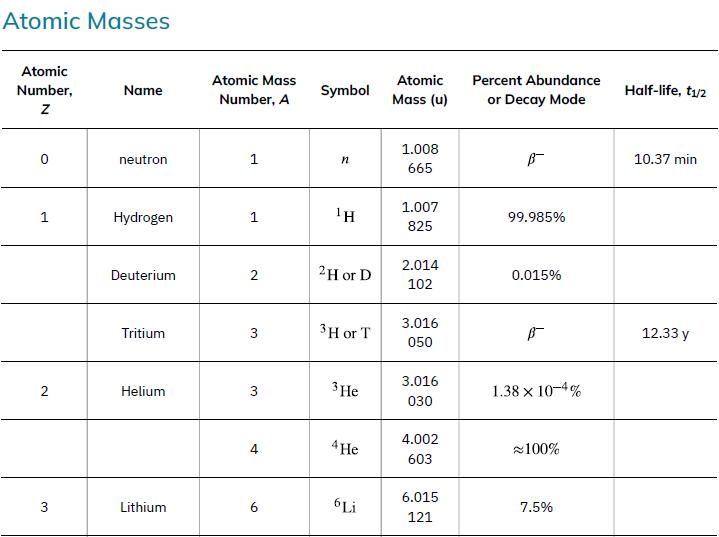
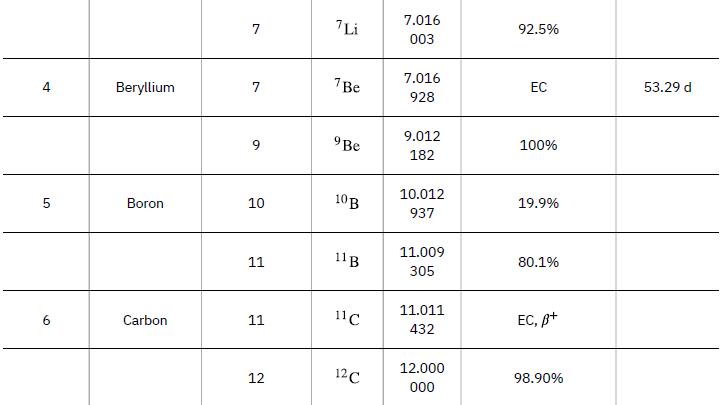
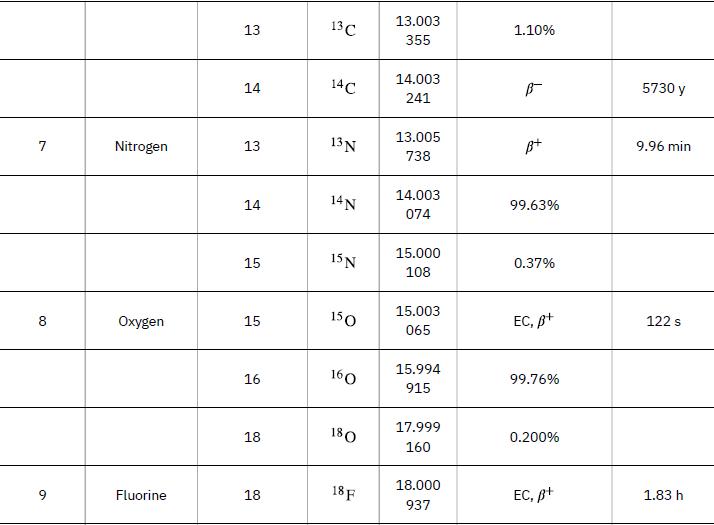
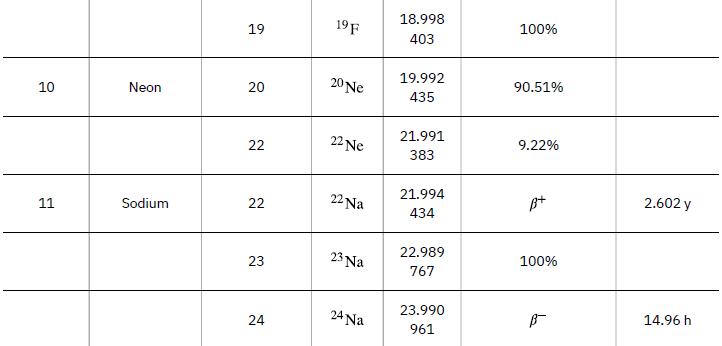
Atomic Masses Atomic Number, Z 1 2 3 Name neutron Hydrogen Deuterium Tritium Helium Lithium Atomic Mass Number, A 1 1 2 3 3 4 9 Symbol ΤΗ 2H or D 3 H or T 3 He 4 He 6 Li Atomic Mass (u) 1.008 665 1.007 825 2.014 102 3.016 050 3.016 030 4.002 603 6.015 121 Percent Abundance or Decay Mode B- 99.985% 0.015% B- 1.38 x 10-4% ≈100% 7.5% Half-life, t₁/2 10.37 min 12.33 y
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The decay equation was given earlier for 239 Pu it is Thus the pertinent masses are those of 239 Pu ...View the full answer

Answered By

DEEPTI A.
Hi
I am a Chartered Accountant (Indian CPA) and an MBA Finance with 18+ years of work experience. I am the owner of a small accounting firm. I provide bookkeeping and accounting support to my clients located in different countries. Together with it, I also teach students and help in solving and completing their accounting-related questions and projects.
My core area of expertise lies in teaching students
1) Accountancy
2) Finance
I teach theoretical subjects too but the above 2 are my favorites. My motto is to have an interactive class and design ways and means to make the concept interesting for my students.
Before starting off with teaching I have worked with different MNC's like GECIS, HSBC and IBM and been a part of their Finance, Accounting, and Financial Analytic teams.
So do provide your questions/queries and I'll be more than happy to answer those and hope to help you in whatever manner I can.
All the best in your endeavors!
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physics questions
-
Find the energy emitted in the - decay of 60 Co. Strategy and Concept As in the preceding example, we must first find m, the difference in mass between the parent nucleus and the products of the...
-
One of the waste products of a nuclear reactor is plutonium-239 ( 239 Pu). This nucleus is radioactive and decays by splitting into a helium-4 nucleus and a uranium-235 nucleus ( 4 He + 235 U), the...
-
50 successes in 200 trials when p = 0.2. For the binomial experiments find the normal approximation for the probability of
-
What is the formula for calculating return on investment (ROI)?
-
What is the distinction between general obligation debt and revenue debt? Which one is likely to bear higher interest rates?
-
Redesign the fractionator of Example 6.8 for a reflux ratio that is twice the minimum. Determine the diameter of the tower, the height of packing in the stripping and rectifying sections, and the...
-
White Way Inc. produces and sells theater set designs and costumes. The company began operations on January 1, 2012. The following transactions relate to securities acquired by White Way Inc., which...
-
A bank in Toronto charges 2.2% commission to buy and sell currencies. Assume that the current exchange rate is US$1 = C$1.1153. a. How many Canadian dollars will you have to pay to purchase US$4,500?...
-
(a) For carbon, calculate the energy when an electron falls from n = 3 to n = 2, then from n = 2 to n = 1. Then add these for the total energy released in the process. (b) Calculate the energy...
-
The solar corona is so hot that most atoms in it are ionized. Consider a hydrogen-like atom in the corona that has only a single electron. Construct a problem in which you calculate selected spectral...
-
{[(C B) (Z A)] [ (B Y) (X Z)]} Determine the truth values of the following symbolized statements. Let A, B, and C be true and X, Y, and Z be false. Circle your answer.
-
Federated Tools, Inc., charges Jacks Hardware five cents per item and Eves Home Store ten cents per item for the same product. Jacks Hardware and Eves Home Store are competitors. If this practice...
-
Auto Corporation makes cars in the United States. To boost the sales of Auto Corporation and other domestic carmakers, Congress can a. neither set quotas nor tax imports. b. only set quotas on...
-
Digital, Inc., makes supercomputers that feature advanced technology. To inhibit Digitals export of its products to other countries, Congress can a. confiscate all profits on exported supercomputers....
-
Under a force majeure clause, a party may be excused from liability for nonperformance. (True/False)
-
Kenya issues bonds to finance the construction of an international airport. Kenya sells some of the bonds in the United States to Larry. A terrorist group destroys the airport, and Kenya refuses to...
-
How does a simplified employee pension plan differ from a Keogh plan? From a qualified pension plan?
-
Consider a game of poker being played with a standard 52-card deck (four suits, each of which has 13 different denominations of cards). At a certain point in the game, six cards have been exposed. Of...
-
A tennis player hits a ball 2.0 m above the ground. The ball leaves his racquet with a speed of 20.0 m/s at an angle 5.0 above the horizontal. The horizontal distance to the net is 7.0 m, and the net...
-
While a person is walking, his arms swing through approximately a 45 angle in s. As a reasonable approximation, we can assume that the arm moves with constant speed during each swing. A typical arm...
-
Human Biomechanics. The fastest pitched baseball was measured at Typically, a baseball has a mass of 145 g. If the pitcher exerted his force (assumed to be horizontal and constant) over a distance of...
-
An Alabama lumberyard has four jobs on order, as shown in the following table. Today is day 205 on the yards schedule. Job Processing time (days) Due Date A 10 233 B 7 226 C 9 218 D 8 227 First,...
-
Determine the category for each of the audit procedures using the categorizations above. Each category can be used once, more than once, or not at all. Verifying that the accounts payable subsidiary...
-
The author of the article refers to Samsung as having once been a copycat manufacturer. What extent do you consider the creation of the VIP centre to be an example of copycat behaviour?

Study smarter with the SolutionInn App


