Question: A diagram for an open- tube manometer is shown below. If the flask is open to the atmosphere, the mercury levels are equal. For each
A diagram for an open- tube manometer is shown below.
If the flask is open to the atmosphere, the mercury levels are equal. For each of the following situations in which a gas is contained in the flask, calculate the pressure in the flask in torr, atmospheres, and pascals.
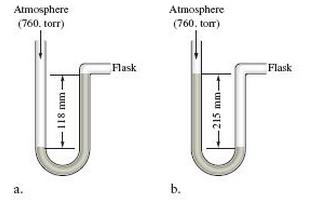
c. Calculate the pressures in the flask in parts a and b (in torr) if the atmospheric pressure is 635 torr.
Atmosphere Atmosphere (760. torr) Atmosphere (760. tom) Flask Flask a. b.
Step by Step Solution
3.51 Rating (184 Votes )
There are 3 Steps involved in it
If the levels of mercury in each arm of the manometer are equal t... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
442-C-P-c-G (2).docx
120 KBs Word File


