Question: A first -order decomposition reaction is observed to have the following rate constants at the indicated temperatures. Estimate the activation energy. k/(10-3 s-1) 2.46 45.1
A first -order decomposition reaction is observed to have the following rate constants at the indicated temperatures. Estimate the activation energy.
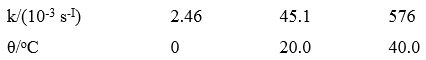
k/(10-3 s-1) 2.46 45.1 576 0/C 20.0 40.0
Step by Step Solution
3.47 Rating (170 Votes )
There are 3 Steps involved in it
As described in Example 225 if the rate constant obeys th... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
57-C-P-C-R (24).docx
120 KBs Word File


