Question: A gas mixture is found to contain two diatomic A and B species for which the partial pressures of both are 0.05065MPa (0.5atm). This mixture
A gas mixture is found to contain two diatomic A and B species for which the partial pressures of both are 0.05065MPa (0.5atm). This mixture is to be enriched in the partial pressure of the A species by passing both gases through a thin sheet of some metal at an elevated temperature. The resulting enriched mixture is to have a partial pressure of 0.02026MPa (0.2atm) for gas A, and 0.01013MPa (0.1atm) for gas B. The concentrations of A and B (CA and CB, in mol/m3) are functions of gas partial pressures (pA2 and pB2, in MPa) and absolute temperature according to the following expressions:?Furthermore, the diffusion coefficients for the diffusion of these gases in the metal are functions of the absolute temperature as follows:
Is it possible to purify the A gas in this manner? If so, specify a temperature at which the process may be carried out, and also the thickness of metal sheet that would be required. If this procedure is not possible, then state the reason(s) why.
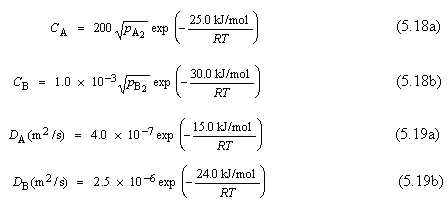
25.0 kJ/mol CA = 200 PA, exp (5.18a) RT 30.0 kJ/mol CB - 1.0 x 10-3 PB, exp (5.186) RT 15.0 kJ/mol DA (m2 /) = 4.0 x 10-7 exp (5.19a) RT 24.0 kJ/mol DE (m2/) = 25 x 10-6 exp (5.196) RT
Step by Step Solution
3.40 Rating (169 Votes )
There are 3 Steps involved in it
This problem calls for us to ascertain whether or not an A 2 B 2 gas mixture may be enriched with re... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (160).docx
120 KBs Word File


