Question: A gas stream containing acetone in air flows from a solvent recovery unit at a rate of 142L/s at 150?C and 1.3 atm. The stream
A gas stream containing acetone in air flows from a solvent recovery unit at a rate of 142L/s at 150?C and 1.3 atm. The stream flows into a condenser which liquefies most of the acetone, and the liquid and gas outlet streams are in equilibrium at ? 18?C and 5.0 atm. Shaft work is delivered to the system at a rate of 25.2 kW to achieve the compression from 1.3 atm to 5.0 atm. To determine the condenser feed stream composition, a 3.00-liter sample of the gas is taken and cooled to a temperature at which essentially all the acetone in the sample is recovered as a liquid. The liquid is poured into an empty flask with a mass of 4.017 g. The flask containing the liquid acetone is weighed and found to have a mass of 4.973 g.
(a) Carry out a degree-of-freedom analysis to show that enough information is available to determine the compositions of all streams and the required heat transfer rate.
(b) Write out a complete set of equations for the molar flow rates of all streams, the mole fractions of acetone in the feed and product gas streams, and the rate (kW) at which heat must be removed in the condenser. Do no calculations.
(c) Solve the equations of part (b) by hand.
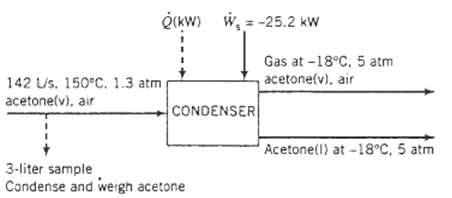
Q(KW) W-25.2 kW 142 Us. 150C. 1.3 atm acetone(v), air CONDENSER 3-liter sample Condense and weigh acetone Gas at -18C. 5 atm acetone(v), air Acetone(l) at -18C, 5 atm
Step by Step Solution
3.41 Rating (170 Votes )
There are 3 Steps involved in it
Let A denote acetone 142 Ls 150C 13 atm no mols yomol Avmol satd 1yomol airmol a Degree of freedom a... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (459).docx
120 KBs Word File


