Question: A gas stream containing acetone in air flows from a solvent recovery unit at a rate of 1 4 2 L s at 1 5
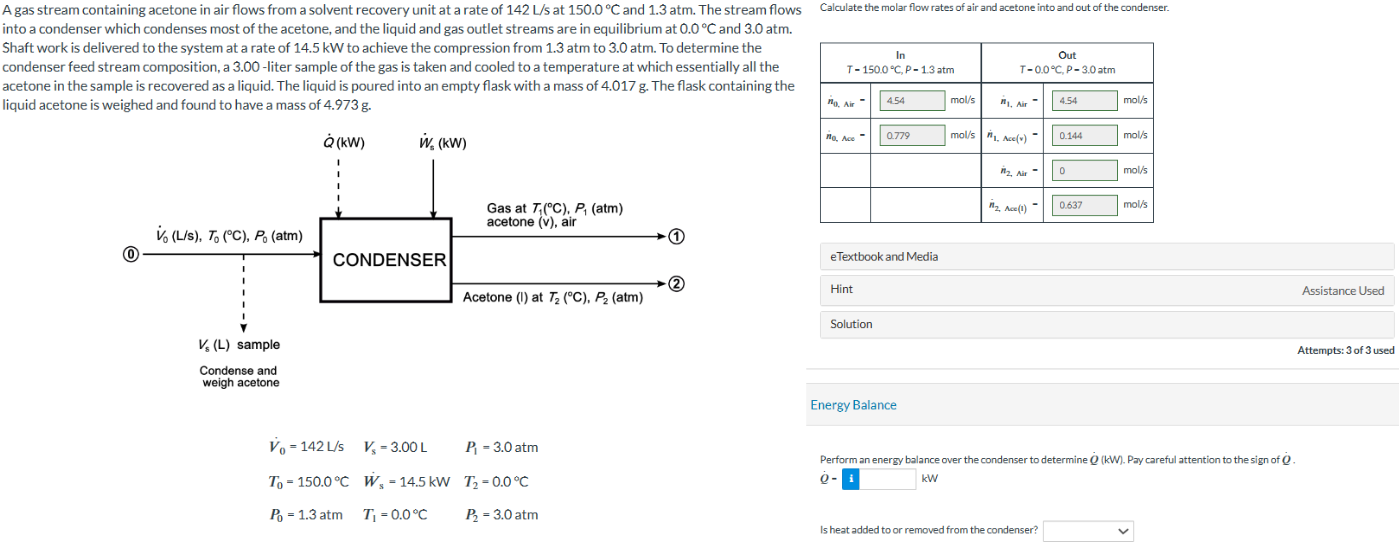
A gas stream containing acetone in air flows from a solvent recovery unit at a rate of at and atm. The stream flows
Calculate the molar flow rates of air and acetone into and out of the condenser.
into a condenser which condenses most of the acetone, and the liquid and gas outlet streams are in equilibrium at and atm.
Shaft work is delivered to the system at a rate of to achieve the compression from atm to atm. To determine the
condenser feed stream composition, a liter sample of the gas is taken and cooled to a temperature at which essentially all the
acetone in the sample is recovered as a liquid. The liquid is poured into an empty flask with a mass of The flask containing the
liquid acetone is weighed and found to have a mass of
eTextbook and Media
Hint
Assistance Used
Solution
Attempts: of used
atm
atm,atm
Perform an energy balance over the condenser to determine Pay careful attention to the sign of Please help me find Q
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


