A group of 35 students attend a class in a room that measures 11 m by 8
Question:
A group of 35 students attend a class in a room that measures 11 m by 8 m by 3 m. Each student takes up about 0.075 m3 and gives out about 80 W of heat (1 W = 1 J/s). Calculate the air temperature rise during the first 20 minutes of the class if the room is completely sealed and insulated. Assume the heat capacity, Cy, for air is 0.718 kJ/(kg K). Assume air is an ideal gas at 20∘C and101.325 kPa. Note that the heat absorbed by the air Q is related to the mass of the air m, the heat capacity, and the change in temperature by the following relationship:
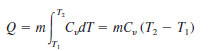
The mass of air can be obtained from the ideal gas law:
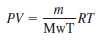
where P is the gas pressure, V is the volume of the gas, Mwt is the molecular weight of the gas (for air, 28.97 kg/kmol), and R is the ideal gas constant [8.314 kPa m3/(kmol K)].
Step by Step Answer:

Numerical Methods for Engineers
ISBN: 978-9352602131
7th edition
Authors: Steven C. Chapra, Raymond P. Canale





