Question: A hydrogen bomb may be approximated by a fireball at a temperature of 7200 K according to a report published in 1950 by the Atomic
A hydrogen bomb may be approximated by a fireball at a temperature of 7200 K according to a report published in 1950 by the Atomic Energy Commission.
(a) Calculate the total rate of radiant-energy emission in watts, assuming that the gas radiates as a blackbody and has a diameter of 1.5 km,
(b) If the surrounding atmosphere absorbs radiation below 0.3μm, determine the per cent of the total radiation emitted by the bomb which is absorbed by the atmosphere,
(c) Calculate the rate of irradiation on a1 m2 area of the wall of a house 40 km from the center of the blast if the blast occurs at an altitude of 16 km and the wall faces in the direction of the blast,
(d) Estimate the total amount of radiation absorbed assuming that the blast lasts approximately 10 sec and that the wall is covered by a coat of red paint,
(e) If the wall were made of oak whose flammability limit is estimated to be 650 K and that had a thickness of 1 cm, determine whether or not the wood would catch on fire. Justify your answer by an engineering analysis stating carefully all assumptions.
GIVEN
- A hydrogen bomb fireball
- Fireball temperature (T1) = 7200 K
- Surrounding atmosphere absorbs radiation below 0.3 ????m
- The blast occurs at an altitude (H) of 16 km = 16,000 m
ASSUMPTIONS
- The gas radiates as a blackbody
- Diameter of the fireball (D) = 1.5 km
- The air and surrounding temperature (T∞) = 10°C
- The surroundings behave as a blackbody enclosure
- The heat transfer from the oak walls to its surroundings during the 10 seconds of irradiation can be neglected
- The house wall is initially at the surroundings temperature
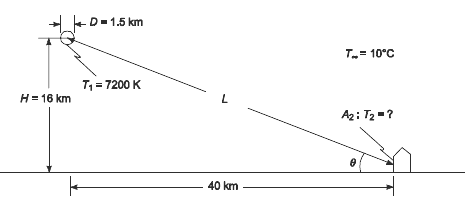
+D-1.5 km T= 10C T1 = 7200 K H= 16 km A2: T2 -? 40 km
Step by Step Solution
3.38 Rating (167 Votes )
There are 3 Steps involved in it
a The total rate of radiation emission is the blackbody emissive power from Equation 93 times the ar... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
66-E-M-E-H-M-T (1931).docx
120 KBs Word File


