Question: For the following half reaction, E cell = 22.07 V: AlF 6 3 + 3e - Al + 6F 2 Using data from Table
For the following half reaction, E°cell = 22.07 V:
AlF63– + 3e- → Al + 6F2
Using data from Table 11.1, calculate the equilibrium constant at 25oC for the reaction Al3+(aq) 1 6F2(aq) ⇌ AlF63–(aq)
Table 11.1
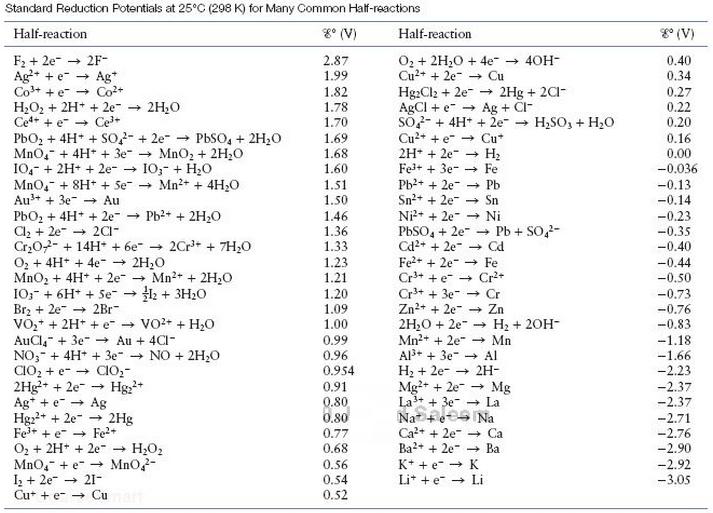
Standard Reduction Potentials at 25G (298 K) for Many Common Haf-reactions Half-reaction %" (V) Half-reaction go (v) Og + 2H2O + 4e- 4OH Cu2+ + 2e- Cu F2 + 2e- 2F- 0.34 0.27 0.22 0.20 1.99 AgCl+ e- Ag+ Cl- SO42-+ 4H' + 2e- H2SO3+ H2O Cu2++ e- Cu" MnO4-+ 4H7 + 3e--> MnO, + 2H20 10c + 2H+ + 2e- io,-+ H,O Mndh" + 8H+ + 5e- Mn2+ 4H2O Fe3++ 3e- Fe Pb2++ 2e- Pb Sn2++ 2e- Sn Ni2++ 2e- Ni -0.036 PbO2 + 4H + + 2e--+ Pb2+ + 2H20 Cl2+ 2e- 2Cl -0.35 Fe2eFe 0, + 4H+ + 4e- 2H2O MnO, 4H+ + 2e- Mn2+ + 2H2O 1.21 1.20 Br2 2e2Br Zn2++ 2e- Zn 2H20 2eH2 20H -0.76 0.99 0.96 Al3eAl H2 2e2H Mg2+ +2e- M 2.23 -2.37 -2.76 -2.92 0.30 La't ter La 0.80 Na e Na + e- A Fe3++ e- Fe2+ Ba2+ + 2e--+ Ba 0.56 Li++ e- Li Cu teCu 0.52
Step by Step Solution
3.40 Rating (181 Votes )
There are 3 Steps involved in it
A1 3 e Al Al 6 F AlF6 3 e ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
442-E-C-E-E-C (33).docx
120 KBs Word File


