Question: Calculate Ksp for iron(II) sulfide given the following data: Es. cell
Calculate Ksp for iron(II) sulfide given the following data:
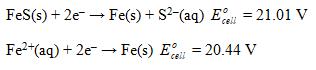
Es. cell
Step by Step Solution
★★★★★
3.49 Rating (175 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The K sp reaction is FeSsFe 2 aq S 2 aq K K sp Manipulate the give... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock
Document Format (1 attachment)
442-E-C-E-E-C (34).docx
120 KBs Word File


