Question: Allenes are compounds with adjacent carboncarbon double bonds. Many allenes are chiral, even though they dont contain chirality centers. Mycomycin, for example, a naturally occurring
Allenes are compounds with adjacent carbon—carbon double bonds. Many allenes are chiral, even though they don’t contain chirality centers. Mycomycin, for example, a naturally occurring antibiotic isolated from the bacterium Nocardia acidophilus, is chiral and has [α]D = – 130. Explain why Mycomycin is chiral. Making a molecular model should be helpful.
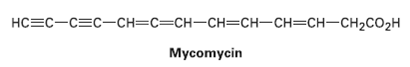
CHCHHCHCHCHCH2CO2H Mycomycin
Step by Step Solution
3.49 Rating (175 Votes )
There are 3 Steps involved in it
Mycomycin contains no chiral carbon atoms yet is chiral To see why make ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-S (189).docx
120 KBs Word File


